Plant Molecular Physiology
Core Course
Staff
Tomonao Matsushita
- Position
- Professor
- Office
- Science Building 2, Room 223
- Phone
- 075-753-4142
- mat(at-mark)gr.bot.kyoto-u.ac.jp
Tomoo Shimada
- Position
- Senior Lecturer
- Office
- Science Building 2, Room 221
- Phone
- 075-753-4140
- tshimada(at-mark)gr.bot.kyoto-u.ac.jp
Yoshito Oka
- Position
- Assistant Professor
- Office
- Science Building 2, Room 221
- Phone
- 075-753-4140
- yoshitooka0302(at-mark)gmail.com
Research
In our laboratory, we are interested in the molecular basis of the amazing ability of plants to adapt to the environment. We focus on processes such as the genome-scale gene expression regulation in response to environmental stimuli, the resulting proteome diversification and intracellular localization of proteins, and long-distance signal transduction between cells, tissues, and organs, studying these phenomena at the gene, protein, and cell levels. We are developing research by a multifaceted approach, making full use of methods such as genomic science, molecular genetics, molecular biology, biochemistry, cell biology, and plant physiology. The specific research subjects are as follows.
1) Transcription start site control by light environment information
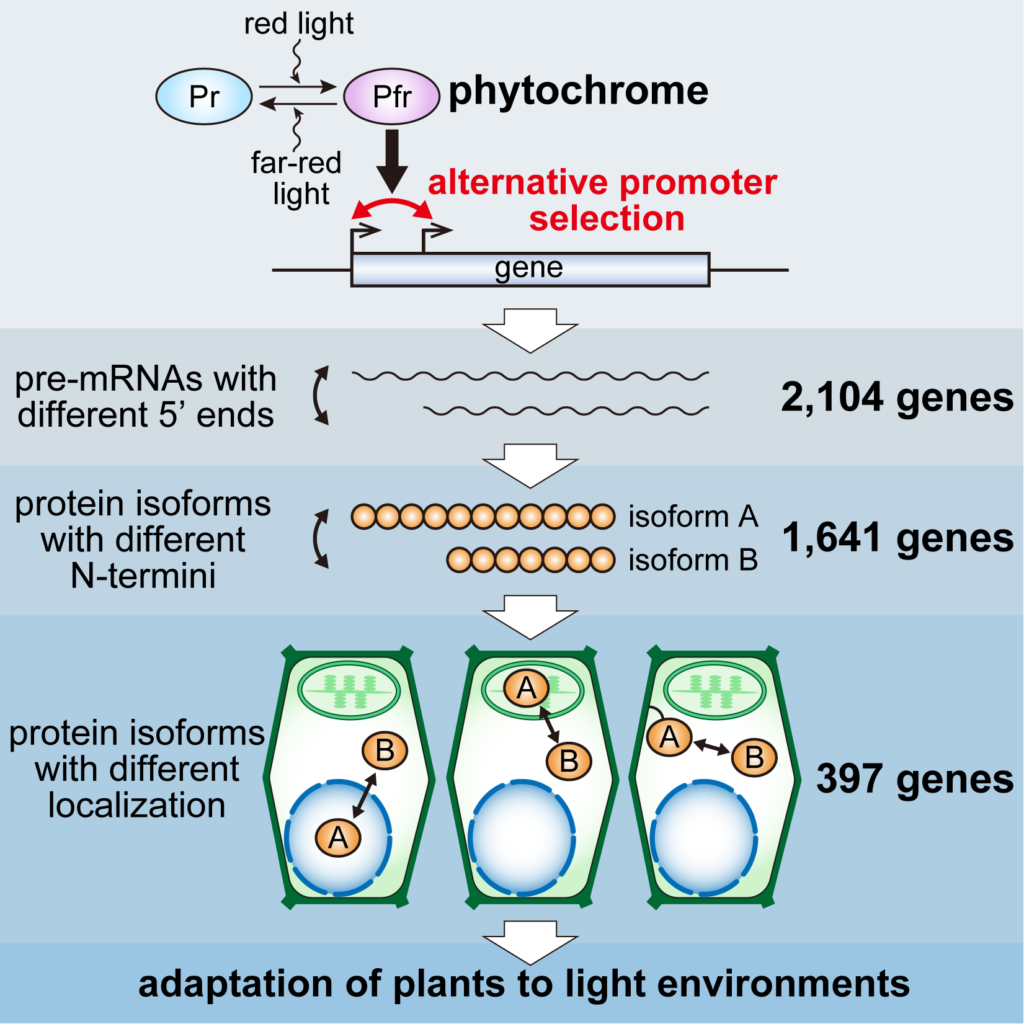
Alternative transcription start site selection is a phenomenon in which mRNA molecules of different lengths are transcribed from multiple transcription start sites existing in a single gene. We recently found that phytochrome, a plant photoreceptor, acts directly on more than 2,000 genes in Arabidopsis to alter their transcription start sites, which in turn alters the intracellular localization of about 400 proteins. We found that these changes in protein localization contribute to the adaptation of plants to various light environments (Ushijima et al., Cell 2017) (Fig. 1).
These discoveries indicate that the phenomenon of alternative transcription start site selection, along with transcription, splicing, and translation, significantly contributes to the functional diversification of proteomes, as a new universal process in the central dogma of eukaryotes. It is likely that transcription start site changes are caused by a common molecular mechanism in eukaryotes due to various signals, not limited to phytochrome signals. Therefore, we use the regulation of transcription start site by phytochrome in Arabidopsis as a model system and elucidate its molecular mechanism to clarify a novel gene expression regulation mechanism universal to eukaryotes, and to add a new process to the central dogma. As a result, it is expected that the textbook of biology will be rewritten in the near future.
In addition, by changing the transcription start site in response to various environmental stimuli, it has become clear that the same single gene produces proteins having functions completely different from those known so far. Therefore, in the future, we will clarify the adaptability of the gene function exerted by switching the transcription start site for more genes, and by uncovering the “back face” of many genes, we will uncover the unexplored region of the proteome.
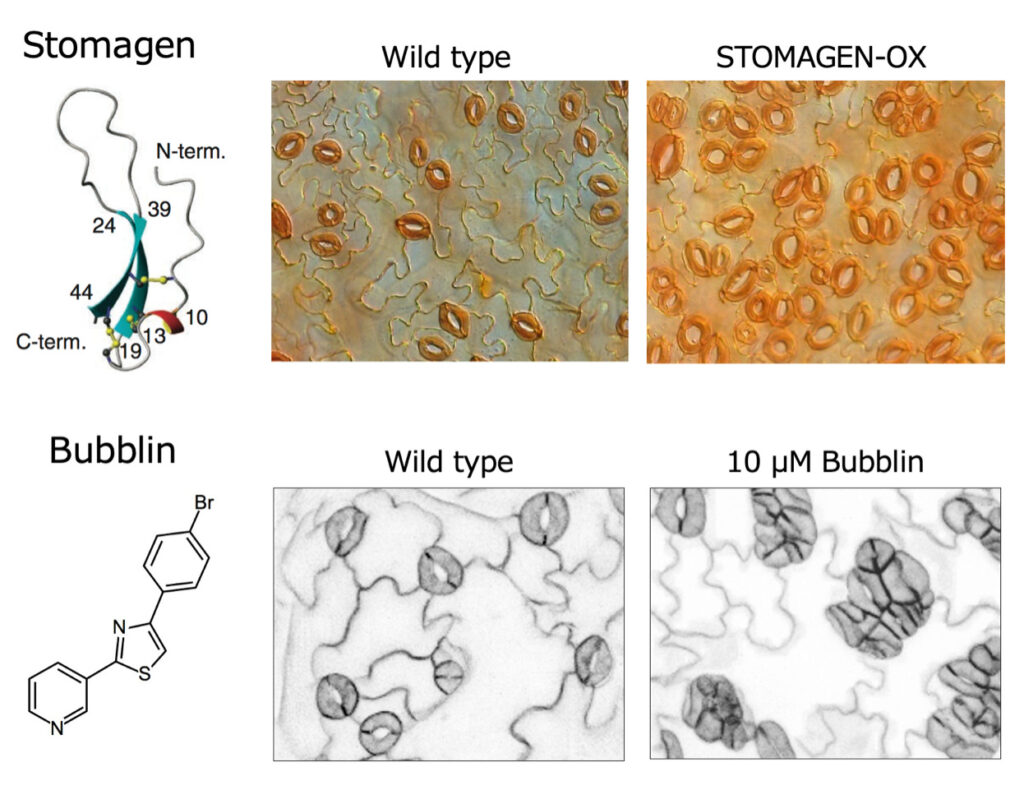
2) Elucidation of the mechanism of stomatal formation
Stomata are important structures that exist on the surface of leaves and function for gas exchange between plants and the atmosphere. We are investigating the mechanism by which stomata are formed using the model plant Arabidopsis thaliana. The Stomagen we discovered is a bioactive peptide consisting of 45 amino acids, an endogenous factor encoded by a plant’s own gene (Sugano et al., Nature 2010) (Fig. 2, top). Stomagen is present in a wide variety of plants, from weeds to crops and trees. Stomagen acts on stomatal precursor cells and has the activity of promoting cell differentiation into guard cells. Since the number of stomata increases when Stomagen is given to plants, it is expected to be useful in applied research that increases the CO2 absorption capacity of various plants.
In addition, we recently discovered a novel compound, Bubblin, that affects stomatal formation (Figure 2, bottom). As a result of screening a chemical library consisting of 3,650 small molecule compounds, it was found that one type of pyridine-thiazole compound affects the stomatal distribution and formation pattern. We named this compound Bubblin and performed an analysis of it. When Bubblin is treated on a plant, many stomata adjacent to each other are formed. Detailed analysis revealed that Bubblin affects the polarity formation of stomatal precursor cells and shows abnormalities in asymmetric cell division. There are many unclear points about the mechanism of polarity formation in plant cells, and it is expected that Bubblin’s analysis will lead to the elucidation of the mechanism that controls the formation of cell polarity in plants.